HSP90: Mechanisms & Interactions
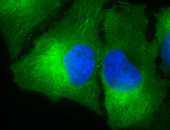
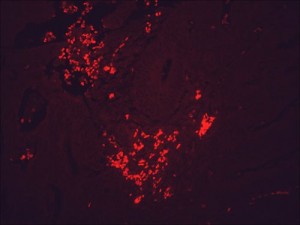 IF detection of Hsp90 in cancerous human colon tissue, using Anti-Hsp90 (clone: H9010)
IF detection of Hsp90 in cancerous human colon tissue, using Anti-Hsp90 (clone: H9010)
HSP90s function as part of multichaperone complexes by interaction with various co-chaperones (co-factors) affecting the binding specificity of Hsp90 for particular client proteins by promoting their conformational integrity. While Hsp90 ensures the stability of these client proteins, its inhibition leads to proteasomal degradation of the clients. Hsp90 clients were originally defined as proteins undergoing degradation after dissociation from Hsp90. Meanwhile, more than 400 clients have been identified up to date and many of them are implicated in mediating signal transduction pathways, apoptotic evasion, differentiation as well as metastasis 142,274,275. A comprehensive list of Hsp90-interacting proteins can be found at the website of Didier Picard. According to their biological function, Hsp90 client proteins can be classified in several groups such as transcription factors, TPR-domain proteins, protein kinases, structural proteins, and others 276. The two main categories, protein kinases and transcription factors, are ubiquitous amongst eukaryotes 72. A further conserved category comprises structurally unrelated mammalian Hsp90 client proteins including viral-replicant proteins as well as receptors involved in innate immune responses 277. In contrast, in yeast this category clusters adapter molecules that play crucial roles as components of the mitochondrial membrane, in vesicular transport processes and secretion 144,278.
The range of Hsp90 co-chaperones also comprise Hsp70 as well as Hsp40 (DnaJ) forming a major cytoplasmic chaperone network. Interaction of Hsp90 with the TPR-domain co-chaperone Hop/Sti-1 leads to the formation of the minimal Hsp90 core complex 168,279. Hop/Sti-1 was shown to completely block the ATPase activity of Hsp90 279, a phenomenon also described for the co-chaperone Cdc-37/p50 which strongly interacts with the NTD of Hsp90 280. The Cdc-37/Hsp90 complex can be formed in an open or closed conformational state, suggesting that Cdc-37 can participate in both, the open encounter and the stable closed complex 281. In contrast, Aha-1 association leads to an increase in the ATPase activity of Hsp90 279, while the co-chaperone p23/Sba-1 couples this ATPase activity to polypeptide dissociation thus functioning as a substrate release factor for Hsp90 282. Amongst the first characterized Hsp90-regulated protein kinases are casein kinase II 244 and Wee-1Swe-1 244. Wee-1Swe-1 has been found to regulate the G2/M transition in the cell cycle through a variety of signal transduction pathways. As already mentioned, Wee-1Swe-1 phosphorylates a conserved tyrosyl residue within the N-terminal domain of Hsp90 which favors the interaction between Hsp90 and several client proteins including Cdk-4, eRBB-2, Raf-1, pp60vsrc, and Wee-1Swe-1 itself 248,283. Further Hsp90 client proteins with kinase activity comprise PKA implicated in Hsp90α secretion and tumour metastasis 140,243, Akt 245, B-Raf 246, and double-stranded DNA-activated protein kinase 247. The latter has been identified to phosphorylate the NTD of human Hsp90α thereby enhancing the association of Hsp90α with the client protein eNOS 243.
Client proteins such as N-Ras and B-Raf are critical components of the MAPK pathway and depend on HSP90 for conformational maturation and folding 284,285. The MAPK pathway is important for regulating cell proliferation and apoptosis 286. Studies clearly demonstrated that Hsp90 is also responsible for stabilizing mutant B-Raf and N-Ras proteins as these molecules are depleted and/or degraded in response to Hsp90 inhibitors 287. When mutated, B-Raf and N-Ras are constitutively active and crucially involved in cell proliferation in several forms of cancer, particularly in melanoma 288,289. It is important to note that Hsp90 is also involved in regulating the key p53 signalling pathways that regulate apoptosis. The TP53 gene encodes the p53 transcription factor which functions as a crucial tumour suppressor by inducing apoptosis that helps to eliminate potential tumour progenitor cells 290,291. Structural analyses of the interaction between Hsp90 and p53 revealed that the CTD of Hsp90 binds directly to the DNA-binding domain of p53 where most of the cancer-causing mutations occur 292,293,294. Studies in stressed fish hepatocytes indicated that up-regulation of Hsp90α activated pro-survival pathways via STAT, ERK-1/2, Akt, ELK-1, Bcl-2, and NF-κB signalling leading to hepatocellular resistance to oxidative stress induced by environmental pollutants, facilitating their survival 295. Concomitantly, the Hsp90α–Akt pathway apparently down-regulated Ask-1 culminating in the inhibition of JNK-1/2 apoptotic signalling. From these findings it becomes apparent that a crosstalk between anti- and pro-apoptotic signalling pathways regulated by Hsp90α modulates the balance between survival and death. HSP90α induction in fish hepatocytes in response to pollutant stress might have provided an adaptative strategy by favouring the signal transduction mechanisms that worked towards survival (STAT, ERK-1/2-ELK-1, Akt/Bcl-2, and NF-κB signalling) and against apoptosis (Ask-1/JNK-1/2 signalling) 295.
Two novel Hsp90 co-chaperones have recently been identified, namely Tah-1 (TPR-containing protein associated with Hsp90) as well as Pih-1 (protein interacting with Hsp90) involved in maturation of small nucleolar ribonucleoprotein particles (snoRNPs) and chromatin remodelling complexes 278,296. Pih-1 and Tah-1 constitute a heterodimeric complex regulating the intrinsic ATPase activity of Hsp90 297. Since the Hsp90-bound heterodimeric Pih-1/Tah-1 complex impacts the assembly of several RNPs, the level of newly synthesized RNPs (U4 small nuclear RNP, telomerase) declines in the presence of geldanamycin accompanied by a loss of certain core proteins (Nop-58, 15.5K) suggesting that Hsp90 may control the folding of these proteins during de novo formation of RNPs 298. A genome-wide analysis on the identification of putative co-factors and substrates of yeast Hsp90 identified the conserved AAA(+)-type DNA helicases Rvb-1 and Rvb-2 as putative interaction partners of Tah-1/Pih-1 thereby constituting the so-called R2TP complex 144. Rvb-1/Rvb-2 are key components of several chromatin remodelling factors thus connecting Hsp90 with epigenetic gene regulation. Recent studies indicate the involvement of RVB-containing complexes in many cellular processes such as transcription, DNA damage response, snoRNP assembly, cellular transformation, and cancer metastasis 144,299.
Amongst the wide panel of transcription factors acting as Hsp90 client proteins, Bcl-6 (B cell lymphoma 6 protein) should be highlighted. Bcl-6 complexed to Hsp90 represses the expression of EP300 encoding the co-activator p300 as well as of BAT3 encoding the p300 co-factor Bat-3 (HLA-B-associated transcript 3). The latter has been shown to form a complex with p300 and to modulate p300-mediated p53 acetylation thus functioning as a novel regulator of the genotoxic stress response 144,300.
There is a wealth of evidence indicating the crucial contribution of Hsp90 to apoptosis and anoikis resistance, crucial processes during carcinogenesis. Hsp90 is crucially involved in inhibiting programmed cell death because it binds directly to Apaf-1 thereby blocking cytochrome c-mediated oligomerization of Apaf-1 and activation of pro-caspase 9 301. Hsp90 inhibits apoptosis through forming a ternary complex with the pro-apoptotic kinase Ask-1 and Akt 302. Hsp90 also exerts anti-apoptotic activity within mitochondria by interacting with Trap-1, cyclophilin D, and survivin. It also blocks the mitochondrial-cytosolic transition of apoptosis-inducing factor (AIF) and endonuclease G 303. According to Whitesell and Lindquist, Hsp90 is able to promote angiogenesis and metastasis by chaperoning certain client proteins including VEGF, NOS, and MMP-2 7. Thus, blockage of Hsp90 expression will consequently trigger apoptosis by suppressing pro-cancerous signalling cascades 7.
The group of Zhijie Chang presents evidence that Hsp90 together with Hsp70 regulates the complex formation of the E3 ubiquitin ligase CHIP with the receptor-dependent TGF-β signalling transducer Smad-3 (son of mother against decapentaplegic 3) 304. This study clearly revealed that Hsp90 inhibits CHIP-mediated Smad-3 ubiquitination and degradation and desensitizes cells in response to TGF-β signalling. Wrighton and collaborators identified TGF-β receptor type I and TGF-β receptor type II as Hsp90-interacting proteins and demonstrated that inhibition of Hsp90 increases TGF-β receptor ubiquitination and degradation dependent on the ubiquitin E3 ligase Smurf-2 305. Since TGF-β signalling is crucially involved in epithelial-mesenchymal transition (EMT), an early event in the multistep process of tumour invasion and metastasis, inhibition of EMT might be a rational strategy to prevent metastasis.
In this context, Hsp90 has been reported to affect stability and proper function of the client proteins c-Met 306,307, the major survival kinase Akt 308 and focal adhesion kinase (Fak) 309. Hsp90 obviously plays an eminent role in the modulation of the extracellular matrix (ECM), since Hsp90β was identified as interaction partner of MMP-3 in the extracellular milieu responsible for mammary epithelial invasion and morphogenesis 310. In conjunction with the co-chaperones Hsp70, Hsp40, Hip, Hop, and p23, extracellular Hsp90α interacts with and promotes the proteolytic activity of MMP-2 in an ATP-independent manner 311,312. It is well known that degradation of ECM acts as a signal for the beginning of invasion and metastasis, and MMPs are important molecules involved in this process 313. As reported previously, Hsp90 and MMP-9 can constitute as a complex in anaplastic large cell lymphomas, and MMP-9 could be activated by Hsp90 to promote cell invasion 314. These data highlight the crucial role of Hsp90 in tumour invasion and metastasis.
Recent studies indicate an involvement of Hsp90 and Hsp70 in the recognition of pathogen-associated molecular patterns (PAMPs) by binding to Toll-like receptor 4 (TLR-4) within lipid rafts 315,316. Since extracellular residing HSP90s are able to stimulate cells of the innate immune system directly and thus act as danger-signalling molecules for the immune system 124,317, these stress proteins have been added to the list of ‘alarmins’. Endogenous alarmins and exogenous PAMPs both comprise the group of danger-associated molecular patterns (DAMPs) 318. Exposure of prostate stromal fibroblasts (PrSFs) to exogenous Hsp90α protein was found to up-regulate the transcription and protein secretion of IL-6 and IL-8, key inflammatory cytokines known to play a causative role in prostate cancer progression 319. Cytokine secretion was regulated in part via a MEK/ERK and NF-κB-dependent pathway. Secreted extracellular Hsp90α also promoted the rapid and durable activation of the oncogenic inflammatory mediator STAT-3, and induced the expression of MMP-3, a well-known mediator of fibrosis and the myofibroblast phenotype 319. Extracellular Hsp90 has also been reported to induce IL8 gene expression in cultured vascular smooth muscle cells in a TLR-4- and NF-κB-dependent manner 320. IL-6 and IL-8 function as key players in carcinogenesis as they are required for initiation of tumour-associated inflammation and neovascularization 321,322. From these observations it can be concluded that Hsp90 is able to enhance the impact of tumourigenic mediators in the tumour microenvironment.
A growing body of evidence now indicates that extracellular residing HSP90s elicit anti-tumour and anti-viral responses by interacting with distinct families of receptors on APCs. As summarized by Binder et al., this interaction has two distinct consequences 323. In the specific or adaptive outcome, HSP-chaperoned peptides are taken up by endocytosing receptors followed by processing and re-presentation to cognate T cells by MHC I molecules on APCs. In the innate or peptide-non specific outcome, HSPs engage surface receptors that trigger the secretion of inflammatory cytokines from APCs via NF-κB activation, up-regulation of co-stimulatory molecules (e.g. MHC II, CD86) followed by migration of DCs to draining lymph nodes 323. Scavenger receptors (e.g. SR-A, CD36, SREC-1)324,325,326 the C-type lectin receptor Lrp1 (CD91/α2-macroglobulin receptor) 327,328, and TLR-2/-4 329 on APCs have been found to facilitate the uptake of exogenous Hsp90. In this context, the class F scavenger receptor SREC-1 binds avidly not only Hsp90 and Grp94 but also Hsp70, Hsp110 and Hsp170 with or without bound antigens indicating its function as a common HSP receptor on APCs 324. Noteworthy, there is a special burdon of proof on molecules suggested to act as putative HSP90 receptors that are also capable of binding LPS (e.g. TLR-2/-4, SR-A, CD36) 323,330.