HSP90: Introduction
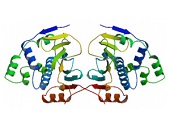
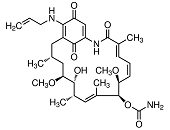
HSP90 proteins define a widespread family of molecular chaperones found in bacteria and all eukaryotes playing a fundamental role in protein homoeostasis and viability. HSP90s primarily exist as homodimers whose activity is regulated by ATP. HSP90 chaperones can be found not only in the cytosol, ER, chloroplasts, mitochondria, and the nucleus 1,2,3 but also in the extracellular milieu where they act as potent stimulators of immune responses 4,5. Hsp90 activity is regulated by post-translational modifications and its association with numerous co-chaperones and client proteins involved in signal transduction and transcriptional regulation. Elevated levels of Hsp90 can be found in a broad spectrum of cancers suggesting a central role in survival and growth of malignant cells 6,7,8. Since numerous oncoproteins are clients of Hsp90, targeting Hsp90 represents a useful anti-cancer approach. Herein, the current knowledge of the Hsp90 chaperone machinery and its role in disease and therapy is compiled.